Chlorine vs Bromine
Controlling microbial growth in cooling towers
Higher cost – higher performance
In an industry where the lowest price is usually a deciding factor, do you really know what you are signing up for? Chlorine is cheaper than bromine so this can make it seem like the obvious choice as a biocide for your cooling tower but what is the science behind choosing a higher cost-higher performance water treatment program?
This is a complex area of study, so we are going to break it down into easy-to-understand components so the trauma of high school chemistry does not come flooding back! Chlorine and bromine, whilst in the same chemical family, have some very notable differences that are critical in providing protection from microbial growth in cooling water systems. Let us look at the key properties of these chemicals side by side.
Chlorine
Liquid chlorine (sodium hypochlorite – the yellow liquid you add to your pool) is relatively cheap and effective at killing bacteria in water. It has been used as a popular disinfectant in water since the late 1800’s and more widely since the 1930’s, continuing until today.
But there is a catch. In order for chlorine to be effective at killing bacteria it needs a low pH water environment and these low pH levels are not present in many cooling water systems. Many systems have limited pH monitoring and correction, and use multiple cycles, which results in slightly alkaline pH levels (higher pH levels of 8.5 to 9), depending on your location and the feedwater quality. This renders the primary biocide ineffective and significantly reduces its ability to disinfect.
Why? Here’s the sciency bit! Once added to water, chlorine (Cl2) will create an initial mixture of hypochlorous acid (HOCl) and hydrochloric acid (HCl):

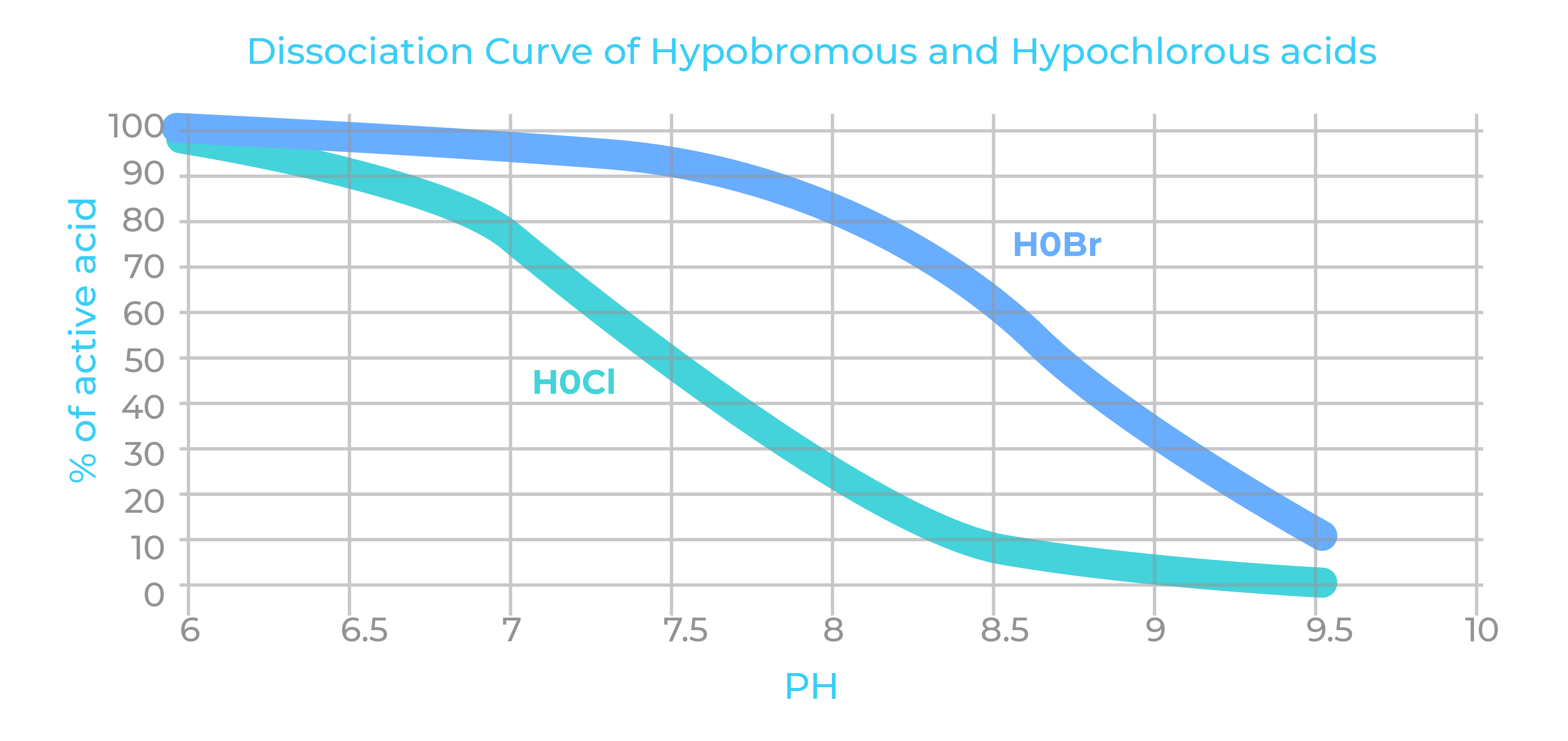
Hypochlorous acid is the powerful sanitizing agent that works to kill microbes. As pH increases (more alkaline) the hypochlorous acid (HOCl) begins to dissociate into its weaker form hypochlorite (OCL-) which is a staggering 80-100 times less effective at killing microbes.
Bromine
Like chlorine, bromine hydrolyzes in water to form hypobromous acid (HOBr), which acts similarly to hypochlorous acid (HOCl). HOBr dissociates to form H+ and OBr-. But here’s where bromine has a major advantage over chlorine. This reaction happens at a much higher pH than chlorine. As a result, a bromine solution in a pH of 8.5 will contain close to 60% of its most effective biocide form (HOBr) whereas a chlorine solution at the same pH would only contain 10% of its most effective biocide form (HOCl).
Another advantage of bromine is that it reacts with ammonia and other nitrogen compounds to form bromamines, which, unlike chloramines, are effective biocides.
In summary, we are not saying chlorine is bad if used in cooling water systems with monitored pH correction, and we know bromine is a more expensive oxidizing biocide than chlorine, however, we believe the advantages and overall effectiveness of bromine offsets the cost in a significant proportion of cooling tower management plans.
Bromine wins hands down
SAS recommend the use of a bromine-based compound because it is far more effective at controlling microbial growth than chlorine.
